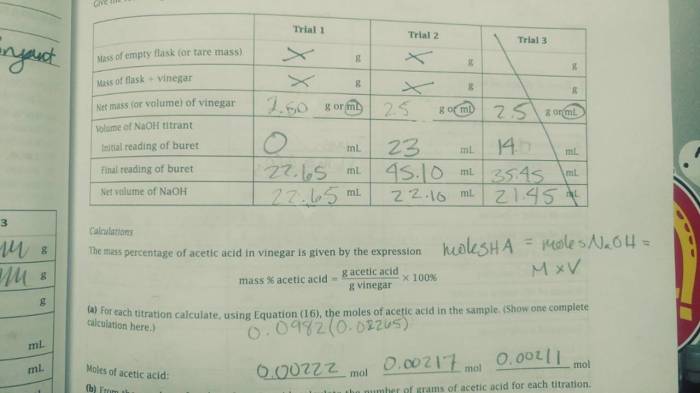
Embarking on an exploration of the molar mass of butane lab, this comprehensive guide delves into the fascinating world of chemistry, unraveling the significance of molar mass in understanding the properties and applications of this versatile hydrocarbon.
Unveiling the intricacies of butane’s molecular structure, we embark on a journey to determine its molar mass, a fundamental property that governs its chemical behavior and opens doors to a multitude of applications.
Introduction to Butane

Butane is a hydrocarbon with the chemical formula C4H10. It is a colorless, flammable gas at room temperature and pressure. Butane is one of the four isomers of butane, the others being isobutane, 2-methylpropane, and 2,2-dimethylpropane.
Physical Properties
Butane has a melting point of
- 138.3 °C, a boiling point of
- 0.5 °C, and a density of 0.578 g/cm³ at 20 °C. Butane is insoluble in water but soluble in organic solvents such as ethanol and ether.
Molar Mass of Butane
Molar mass is a fundamental concept in chemistry that refers to the mass of one mole of a substance. It is a crucial property that helps us understand the composition and behavior of chemical compounds.
To calculate the molar mass of butane, we need to consider its molecular formula, C 4H 10. The molar mass is the sum of the atomic masses of all the atoms in the molecule. The atomic mass of carbon is 12.011 g/mol, and that of hydrogen is 1.008 g/mol.
Steps to Calculate Molar Mass
- Multiply the number of atoms of each element by its respective atomic mass.
- Add up the products obtained in step 1.
For butane:
Molar mass = (4 x 12.011 g/mol) + (10 x 1.008 g/mol) = 58.124 g/mol
Therefore, the molar mass of butane is 58.124 g/mol.
Empirical Formula and Molecular Formula
The empirical formula of a compound represents the simplest whole-number ratio of the elements present. For butane, the empirical formula is CH 2. The molecular formula, on the other hand, represents the actual number of atoms of each element in a molecule.
For butane, the molecular formula is C 4H 10.
The molar mass of a compound can be used to determine its empirical formula if the molecular formula is unknown. By comparing the molar mass of the compound to the molar masses of possible empirical formulas, we can deduce the simplest whole-number ratio of the elements present.
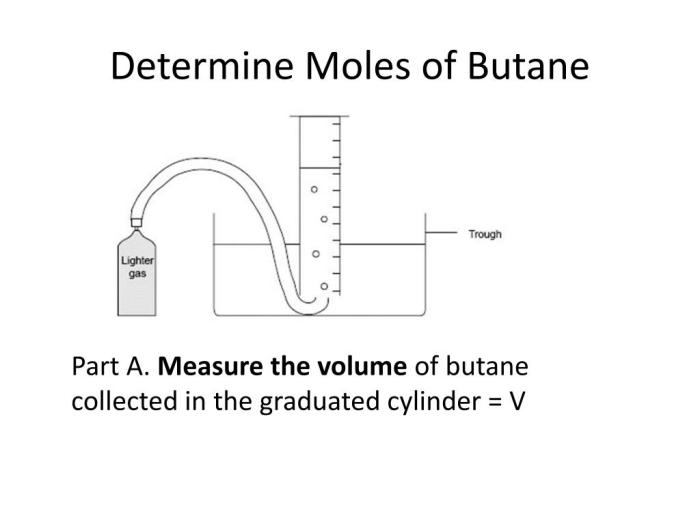
Experimental Procedure for Determining Molar Mass
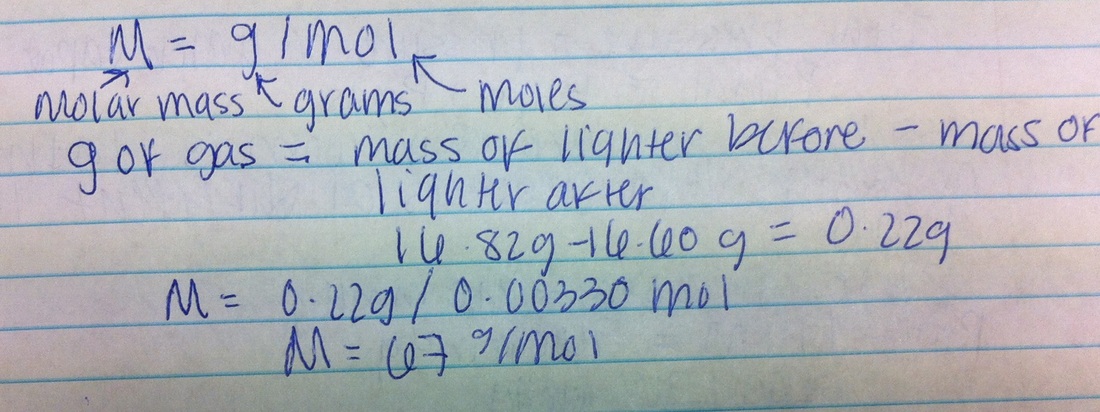
Determining the molar mass of butane involves a meticulous experimental procedure that employs either combustion analysis or gas chromatography. Both techniques rely on specific principles and require specialized equipment and materials.
Combustion Analysis
Combustion analysis is a widely used technique for determining the molar mass of hydrocarbons like butane. The experiment involves combusting a known mass of the sample in a controlled environment, measuring the volume of carbon dioxide produced, and calculating the molar mass based on the stoichiometry of the combustion reaction.
- Experimental Setup:The experimental setup consists of a combustion chamber, a gas burette, and a precision balance.
- Procedure:A weighed sample of butane is placed in the combustion chamber and ignited. The combustion products, including carbon dioxide, are collected in the gas burette, and their volume is measured. The mass of carbon dioxide produced is calculated, and the molar mass of butane is determined using the stoichiometry of the combustion reaction: C 4H 10+ 6.5O
If you’re wondering if you’re being bullied, take this am I being bullied quiz . It can help you identify the signs and symptoms of bullying. If you’re concerned about the molar mass of butane, remember that it’s 58.12 g/mol.
2→ 4CO 2+ 5H 2O.
Gas Chromatography
Gas chromatography is another technique employed to determine the molar mass of butane. This method involves separating the components of a gas sample based on their different affinities for a stationary phase. The sample is injected into a gas chromatograph, which separates the components and detects their presence using a detector.
- Experimental Setup:The experimental setup consists of a gas chromatograph equipped with a column, a carrier gas, and a detector.
- Procedure:A sample of butane is injected into the gas chromatograph, and the components are separated as they pass through the column. The detector measures the concentration of butane in the eluent, and the molar mass is determined by comparing the retention time of butane to the retention times of known standards.
Data Analysis and Calculations: Molar Mass Of Butane Lab
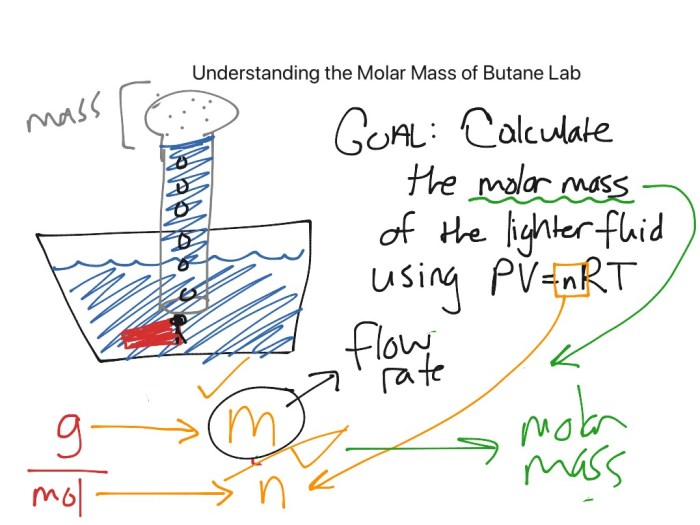
The experimental data collected during the butane molar mass determination can be analyzed using various methods to calculate the molar mass of butane.
One common method involves plotting a graph of the mass of butane used versus the volume of air displaced. The slope of the graph represents the molar mass of butane.
Another method involves using statistical analysis to determine the mean and standard deviation of the experimental data. The mean value represents the molar mass of butane, while the standard deviation provides an estimate of the uncertainty in the measurement.
Potential Sources of Error, Molar mass of butane lab
There are several potential sources of error that can affect the accuracy of the molar mass determination. These include:
- Inaccurate measurement of the mass of butane used
- Inaccurate measurement of the volume of air displaced
- Incomplete combustion of butane
- Presence of impurities in the butane sample
To minimize the impact of these errors, it is important to use accurate measuring equipment, carefully follow the experimental procedure, and use a pure butane sample.
Applications of Molar Mass
The molar mass of butane plays a crucial role in various chemical applications. It enables chemists and scientists to determine the molecular weight of compounds, calculate stoichiometric ratios, and predict chemical properties.
One of the primary applications of molar mass is in determining the molecular weight of compounds. By knowing the molar mass of butane, chemists can calculate the molecular weight of other compounds by comparing their relative masses. This information is essential for identifying and characterizing chemical substances.
Calculating Stoichiometric Ratios
The molar mass of butane is also used to calculate stoichiometric ratios in chemical reactions. Stoichiometry refers to the quantitative relationship between reactants and products in a chemical reaction. By knowing the molar mass of butane, chemists can determine the exact amount of reactants and products involved in a reaction, ensuring efficient and accurate experimentation.
Predicting Chemical Properties
Furthermore, the molar mass of butane provides insights into the chemical properties of compounds. For instance, compounds with higher molar masses tend to have higher boiling points and lower vapor pressures. This knowledge aids in predicting the physical and chemical behavior of substances, guiding research and development in various fields.
Top FAQs
What is the molar mass of butane?
The molar mass of butane is 58.12 grams per mole.
How do you calculate the molar mass of butane?
To calculate the molar mass of butane, add the atomic masses of the constituent atoms. The atomic mass of carbon is 12.011 grams per mole, and the atomic mass of hydrogen is 1.008 grams per mole. Thus, the molar mass of butane (C4H10) is (4 x 12.011) + (10 x 1.008) = 58.12 grams per mole.
What are the applications of molar mass?
The molar mass of a substance is used in various applications, including determining the molecular weight of compounds, calculating stoichiometric ratios, and predicting chemical properties.