How many chirality centers does the following molecule contain? This question lies at the heart of understanding molecular chirality, a fundamental concept in chemistry that plays a crucial role in drug design and other fields. This article delves into the intricacies of chirality centers, providing a comprehensive guide to identifying and determining their number in a given molecule.
Chirality centers, also known as stereogenic centers, are atoms within a molecule that give rise to stereoisomerism. Stereoisomers are molecules with the same molecular formula but different spatial arrangements of their atoms. The number of chirality centers in a molecule directly influences the number of stereoisomers it can possess.
1. Define Chirality Center
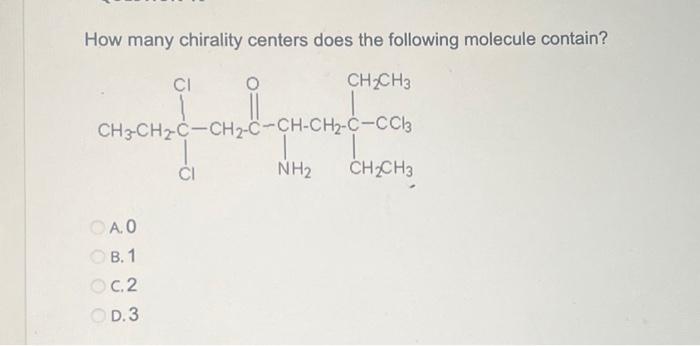
A chirality center is a carbon atom that is bonded to four different groups. The presence of a chirality center makes a molecule chiral, meaning it cannot be superimposed on its mirror image.
Examples of molecules with chirality centers include:
- Glyceraldehyde
- Lactic acid
- Ibuprofen
Examples of molecules without chirality centers include:
- Methane
- Ethane
- Benzene
2. Identify Chirality Centers: How Many Chirality Centers Does The Following Molecule Contain
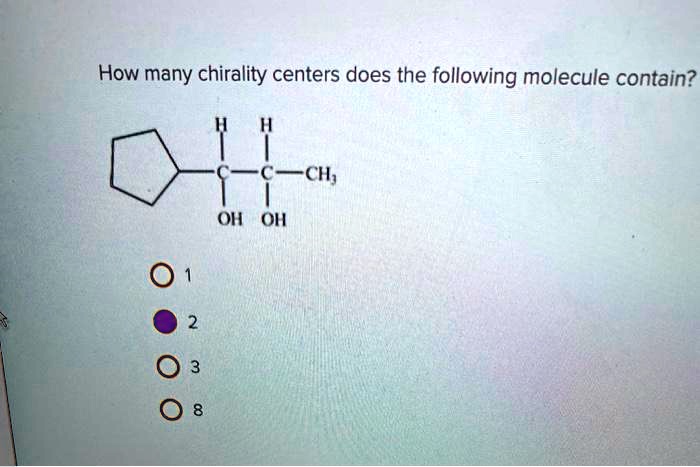
To identify chirality centers in a molecule, look for carbon atoms that are bonded to four different groups. The groups can be atoms, functional groups, or other molecules.
The relationship between the number of chirality centers and the number of stereoisomers is given by the following equation:
N = 2n
where N is the number of stereoisomers and n is the number of chirality centers.
3. Determine the Number of Chirality Centers
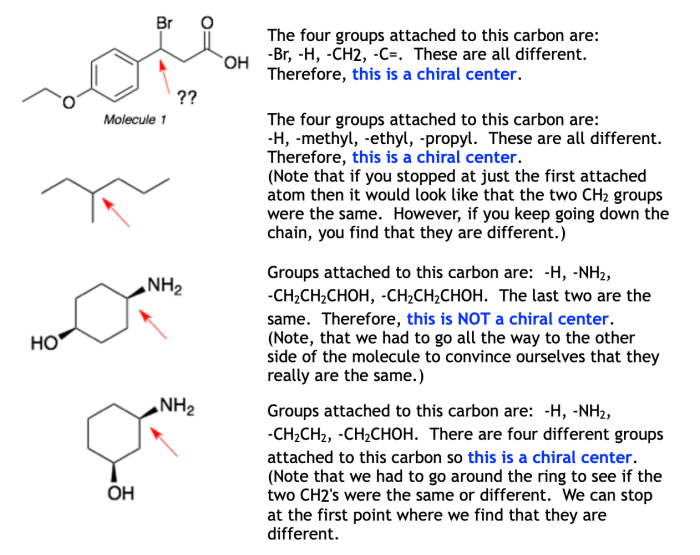
To determine the number of chirality centers in a molecule, follow these steps:
- Identify all of the carbon atoms in the molecule.
- For each carbon atom, count the number of different groups that are bonded to it.
- If a carbon atom is bonded to four different groups, then it is a chirality center.
- Count the number of chirality centers in the molecule.
The following table illustrates the process for determining the number of chirality centers in different types of molecules:
| Molecule | Number of Carbon Atoms | Number of Different Groups Bonded to Each Carbon Atom | Number of Chirality Centers |
|---|---|---|---|
| Methane | 1 | 4 | 0 |
| Ethane | 2 | 4 | 0 |
| Glyceraldehyde | 3 | 4 | 1 |
| Lactic acid | 4 | 4 | 1 |
| Ibuprofen | 15 | 4 | 2 |
4. Exceptions and Considerations
There are some exceptions to the general rules for determining the number of chirality centers. For example, if a carbon atom is bonded to two identical groups, then it is not a chirality center. This is because the two identical groups cannot be distinguished from each other.
Symmetry can also affect the number of chirality centers in a molecule. For example, a molecule with a plane of symmetry has no chirality centers, even if it has carbon atoms that are bonded to four different groups.
5. Applications of Chirality
Chirality is an important concept in drug design and other fields. For example, the enantiomers of a drug can have different biological activities. This is because the enantiomers can interact with different receptors in the body.
Examples of how chirality can impact the biological activity of molecules include:
- The enantiomer of thalidomide that caused birth defects was the S-enantiomer.
- The enantiomer of ibuprofen that is used to relieve pain is the S-enantiomer.
- The enantiomer of warfarin that is used to prevent blood clots is the S-enantiomer.
Essential Questionnaire
What is a chirality center?
A chirality center is an atom within a molecule that is bonded to four different groups, resulting in a non-superimposable mirror image.
How do I identify chirality centers in a molecule?
To identify chirality centers, examine each carbon atom in the molecule. If the carbon is bonded to four different groups, it is a potential chirality center.
What is the relationship between the number of chirality centers and the number of stereoisomers?
The number of stereoisomers for a molecule is 2^n, where n is the number of chirality centers.